NET-dependent metastasis regulated by a transmembrane DNA receptor was revealed by Erwei Song’s team
On June 11th, Dr Erwei Song’s team from Sun Yat-sen Memorial Hospital, Sun Yat-sen University, published a research paper entitled "DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25" online in Nature. The study identifies a transmembrane DNA receptor CCDC25 that mediates NET-dependent metastasis. Dr. Linbing Yang is the first author of this article. Dr Erwei Song and Dr Shicheng Su are the co-corresponding authors.
As we all know, tumor cells just like "seeds", their growth are relied on the fertile soil, that is, the tumor microenvironment. However, it remains unclear how the tumor cells interact with distant organ tissues to promote the distant organ metastases.
Neutrophil extracellular traps (NETs), which consist of chromatin DNA filaments coated with granule proteins, are released by neutrophils to trap microorganisms. Recent studies have suggested that the DNA component of NETs (NET-DNA) is associated with cancer metastasis in mouse models. However, the functional role and clinical importance of NET-DNA in metastasis in patients with cancer remain unclear.
To investigate the clinical importance of NETs, we conducted immunofluorescence staining for myeloperoxidase (MPO) and citrullinated histone H3 (H3Cit)—specific markers for neutrophils and NETosis, respectively—in the primary tumor and the metastatic lesions of breast cancer patients. The study showed that liver metastases exhibited the most abundant NET infiltration. Besides, in patients with breast cancer, higher levels of serum MPO–DNA was an independent variable associated with subsequent metastasis to the live. Together, these data implied that excessive amounts of NETs could form in the livers of patients with breast cancer before metastases could be detected, and could facilitate the subsequent development of liver metastases.
To determine whether NETosis in the distant organs preceded cancer metastasis, the study constructed a breast cancer liver metastasis model by in situ injection and intrasplenical injection of cancer cells. Abundant NETs were observed in the livers before detectable metastasis and increasing during the liver metastasis cascades. More importantly, inhibition of NET formation could significantly reduce the liver metastasis in vivo.
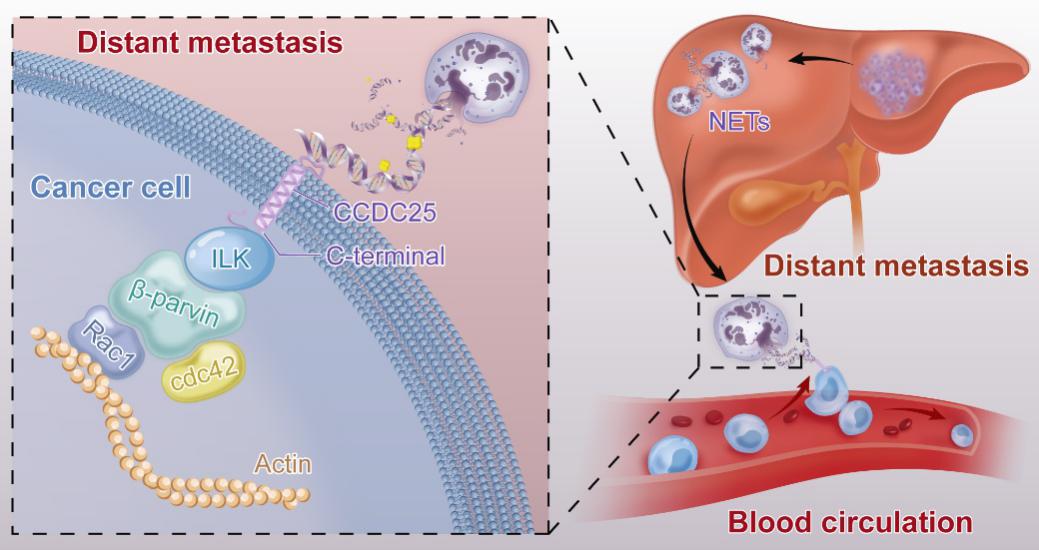
Mechanically, the study found that NET-DNA acted as a chemotactic factor to attract cancer cells, rather than merely acting as a ‘trap’ for them. In several mouse models, NETs in the liver or lungs were found to attract cancer cells to form distant metastases. Furthermore, they identified the transmembrane protein CCDC25 as a NET-DNA receptor on cancer cells that sensed extracellular DNA and subsequently activated the ILK–β-parvin pathway to enhance cell motility.
To investigate the role of CCDC25 in more clinically relevant models, the study generated CCDC25-knockout mice and crossed them with MMTV-PyMT mice to generate PyMT;CCDC25−/− hybrids and injected the breast tumor cells isolated from PyMT and PyMT;CCDC25−/− mice into the spleens of syngeneic C57BL/6 mice and observed that CCDC25 depletion significantly inhibited liver metastases. Besides they isolated primary cancer cells from breast cancer patients and knocked out CCDC25 using CRISPR–Cas9 techniques. they found that CCDC25 knockout significantly inhibited liver metastases of the primary tumor cells that were intrasplenically injected into NOD/SCID mice. More notably, they observed that the CCDC25 antibody markedly inhibited the formation of liver metastases when MDA-MB-231 cells were injected into the spleens of NOD/SCID mice. Overall, the study identifies a transmembrane DNA receptor that mediates NET-dependent metastasis, and suggests that targeting CCDC25 could be an appealing therapeutic strategy for the prevention of cancer metastasis.
Hyperlink of the article:https://www.nature.com/articles/s41586-020-2394-6


