Novel Strategy by Yixian Team to Prevent Severe Capecitabine-Induced Hand-Foot Syndrome Published in The BMJ
On September 11, 2025, The BMJ published a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial led by Professor Gong Chang’s team at Sun Yat-sen Memorial Hospital (SYSMH), in collaboration with six other Chinese institutions. The study, entitled "Effect of methylcobalamin on capecitabine induced hand-foot syndrome in patients with HER2 negative early breast cancer," demonstrated that oral mecobalamin significantly reduced the incidence of grade 2/3 hand-foot syndrome (HFS) in patients receiving adjuvant capecitabine, with no notable adverse reactions, thereby offering an effective and safe prophylactic strategy for HFS in this patient population.
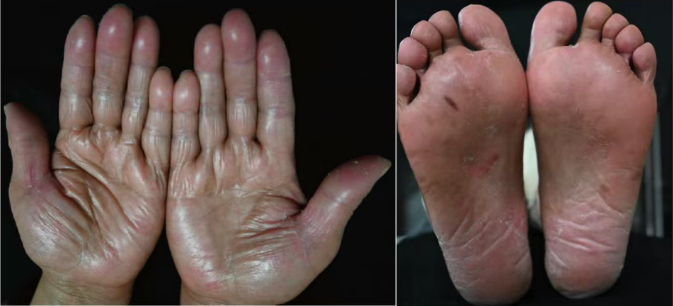
Illustrative Images of Severe Hand-Foot Syndrome
HFS is one of the most common adverse reactions associated with capecitabine. While not life-threatening, grade 2/3 HFS substantially impairs quality of life and frequently necessitates clinical interventions such as dose reduction or treatment discontinuation, which ultimately compromise therapeutic efficacy and survival outcomes. Given the current lack of safe and effective preventive measures, the exploration of novel prophylactic strategies is urgently needed.
Current clinical strategies for the prevention of capecitabine-induced HFS include celecoxib, topical diclofenac, and urea-based cream. However, each of these interventions has significant limitations: long-term celecoxib use is associated with an elevated risk of cardiovascular events; evidence supporting topical diclofenac is insufficient, as clinical studies have assessed its efficacy only on the hands rather than comprehensive HFS; and while urea cream can reduce the incidence of grade 1 HFS, its prophylactic effect against more severe grade 2/3 HFS remains unestablished. Consequently, due to these limitations, clinical guidelines assign a low evidence level and recommendation strength (at most grade IIB) to these agents for preventing capecitabine-induced HFS. This underscores the persistent clinical need for safer and more effective prophylactic approaches.
Methylcobalamin, a vitamin B12 derivative, promotes neurite outgrowth and neuronal survival by enhancing Erk1/2 and Akt activities via the methylation cycle. These mechanisms alleviate neuropathic pain and dysesthesia and improve small fiber neuropathy, indicating methylcobalamin's potential for preventing capecitabine-induced HFS.
To investigate this premise, the research team conducted a clinical trial enrolling 234 patients with HER2-negative early breast cancer receiving adjuvant capecitabine. Participants were randomized to receive either methylcobalamin (n=117) or a placebo (n=117).
The results showed that the incidence of grade 2/3 HFS was significantly lower in the methylcobalamin group than in the placebo group (14.5% [17/117] vs. 29.1% [34/117]; rate difference: −14.5%, 95% CI: −24.9% to −4.1%). The incidence of other treatment-related adverse events was comparable between groups: 75.2% (88/117) in the methylcobalamin group and 81.2% (95/117) in the placebo group. No unique adverse reactions to methylcobalamin were identified. Furthermore, the proportion of patients requiring capecitabine dose reduction or discontinuation due to HFS was lower with methylcobalamin (7.7% vs. 13.7%).
In clinical practice, capecitabine is used not only as an adjuvant intensification therapy for HER2-negative early breast cancer but also widely for advanced breast cancer and other solid tumors, including gastric, esophageal, and colorectal cancers. This study provides robust evidence for managing HFS in patients receiving capecitabine-based regimens. By offering an effective prophylactic strategy, it helps ensure patients can maintain adequate dosage and complete full-course treatment, thereby enhancing therapeutic efficacy and safeguarding patient outcomes. Furthermore, these findings may provide high-quality clinical evidence to inform updates to relevant preventive guidelines.

Research Summary Diagram
About the Corresponding Author

Prof. Gong Chang is a Chief Physician of Breast Surgery, PhD supervisor, and Director of the Breast Diagnosis Centre at SYSMH. She holds several key national appointments, including Standing Committee Member of the Integrated Prevention and Screening Committee of the Chinese Anti-Cancer Association (CACA) Breast Cancer Committee, Member of the CACA Breast Cancer Committee, Standing Committee Member of the Breast Cancer Committee of the Chinese Women Medical Doctors' Association, and Member of the Oncology Working Group under the Medical Geneticist Branch of the Chinese Medical Doctor Association.
She has a distinguished record of securing competitive research funding, serving as Principal Investigator for numerous grants. Her portfolio includes one National Key R&D Program of China grant, one Major National Science and Technology Program grant, seven grants from the National Natural Science Foundation of China (NSFC), seven ministerial/provincial-level grants, and two from the Chinese Society of Clinical Oncology (CSCO) Foundation. Her research contributions have been recognized with five prestigious talent awards, notably including the Distinguished Young Scholar title from the Ministry of Science and Technology, the Ministry of Education, and Guangdong Province.
She served as chief editor for the monograph Application of Tissue Markers in Precision Diagnosis and Treatment of Breast Diseases (People's Medical Publishing House). Her award-winning research, which secured the Second Prize of the Guangdong Provincial Scientific and Technological Progress Award (as first accomplisher), has been cited in the Chinese Expert Consensus on Young Breast Cancer and 17 textbooks/monographs, including the postgraduate texts Fundamentals of Pathology and Oncology.
In addition to her technological contributions—including 12 patents and the development of two software programs—Prof. Gong has a substantial publication record. As corresponding author (including co-corresponding), she has published 35 papers in prestigious journals such as The BMJ, Molecular Cancer, Advanced Science, Cell Reports Medicine, Med, and The Oncologist. These works have accumulated nearly 3,000 citations, four of which are classified as highly cited papers.


